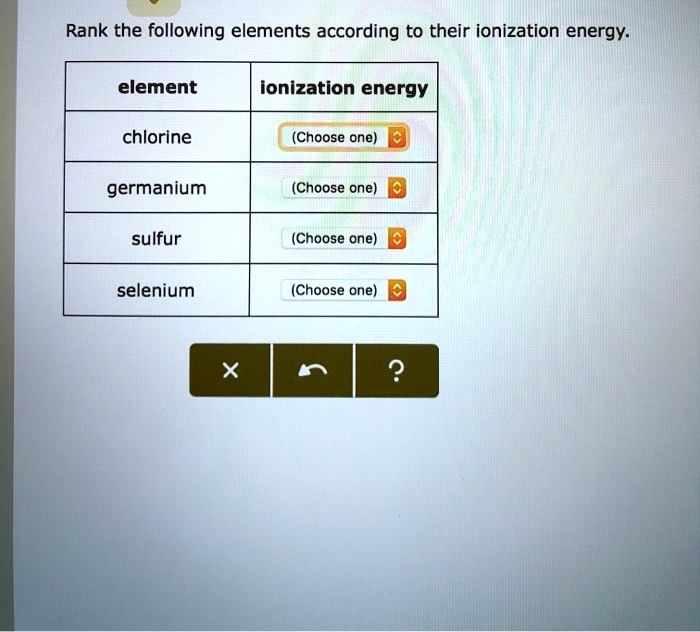
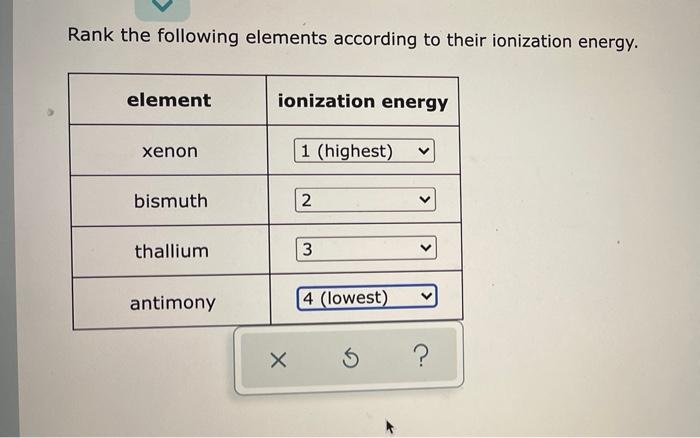
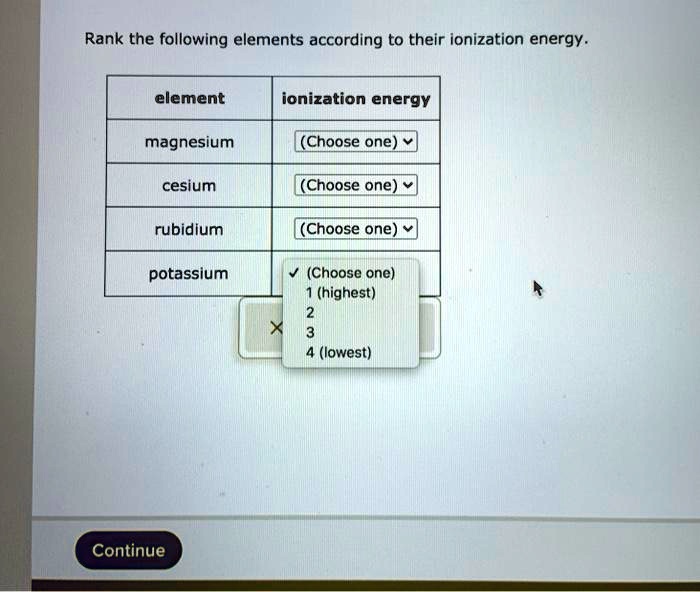
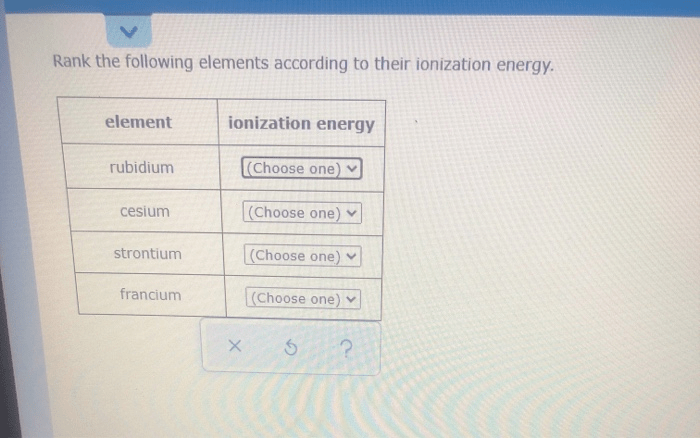
Rank the following elements according to their ionization energy. Ionization energy is the minimum amount of energy required to remove an electron from an atom or ion in its gaseous state. It is a key concept in chemistry and physics, as it provides insights into the electronic structure of atoms and their reactivity.
In this article, we will explore the factors affecting ionization energy, its periodic trends, applications, and exceptions to the general trends.
The ionization energy of an element is influenced by several factors, including its atomic radius, nuclear charge, and electron configuration. The periodic trends in ionization energy can be explained by the Aufbau principle and the increasing nuclear charge across a period and down a group.
Ionization energy plays a crucial role in understanding chemical bonding, reactivity, and various applications in chemistry and physics.
Rank the Following Elements According to Their Ionization Energy
Ionization energy is the energy required to remove an electron from an atom in its gaseous state. It is a fundamental property of an element that provides insights into its chemical reactivity and behavior. The following table ranks the ionization energies of various elements:| Element | Ionization Energy (kJ/mol) ||—|—|| Hydrogen | 1312 || Helium | 2372 || Lithium | 520 || Beryllium | 900 || Boron | 801 || Carbon | 1086 || Nitrogen | 1402 || Oxygen | 1314 || Fluorine | 1680 || Neon | 2081 |
Commonly Asked Questions: Rank The Following Elements According To Their Ionization Energy.
What is the formula for calculating ionization energy?
The formula for calculating ionization energy (IE) is: IE = -ΔH/n, where ΔH is the enthalpy change of the ionization process and n is the number of electrons removed.
Why does ionization energy increase across a period?
Ionization energy increases across a period because the increasing nuclear charge attracts the electrons more strongly, making it more difficult to remove an electron.
What are the applications of ionization energy?
Ionization energy is used in various applications, including mass spectrometry, plasma physics, and understanding chemical bonding and reactivity.