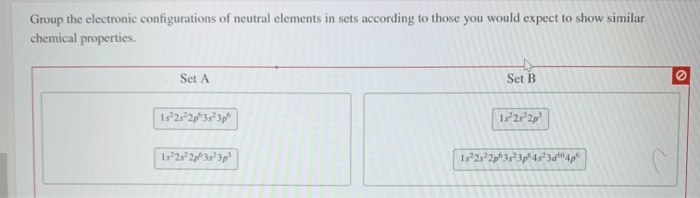
The study of group the electronic configurations of neutral elements in sets unveils the fundamental principles that govern the organization and behavior of elements in the periodic table. By examining the patterns and similarities in their electron arrangements, we gain insights into their chemical properties, reactivity, and the formation of various compounds.
This exploration delves into the periodic trends observed in electronic configurations, the different approaches to grouping elements based on these configurations, and the unique characteristics of specific groups, including noble gases, alkali metals, halogens, and transition metals.
Periodic Trends in Electronic Configurations: Group The Electronic Configurations Of Neutral Elements In Sets
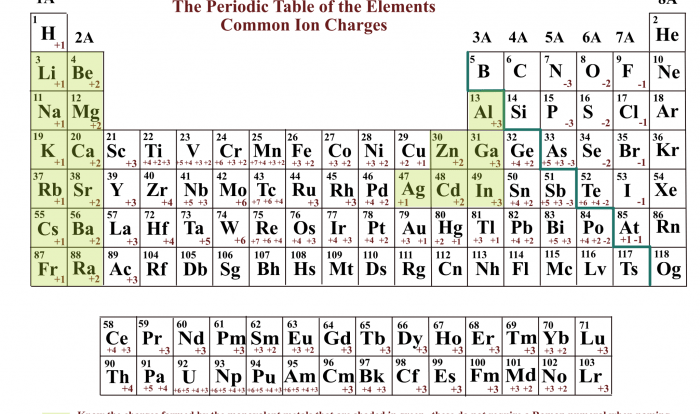
The electronic configurations of elements exhibit periodic trends, meaning that certain patterns and regularities can be observed as we move across the periodic table. These trends provide valuable insights into the chemical properties and behavior of elements.
One of the most prominent periodic trends is the increase in the number of electrons in the outermost energy level, known as the valence electrons, as we move from left to right across a period. This trend directly influences the chemical reactivity of elements.
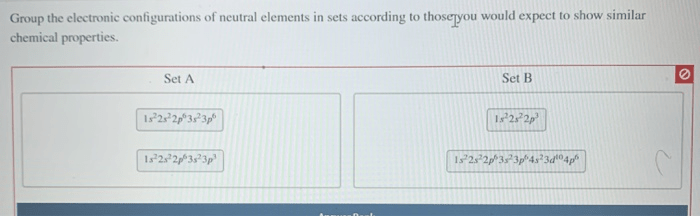
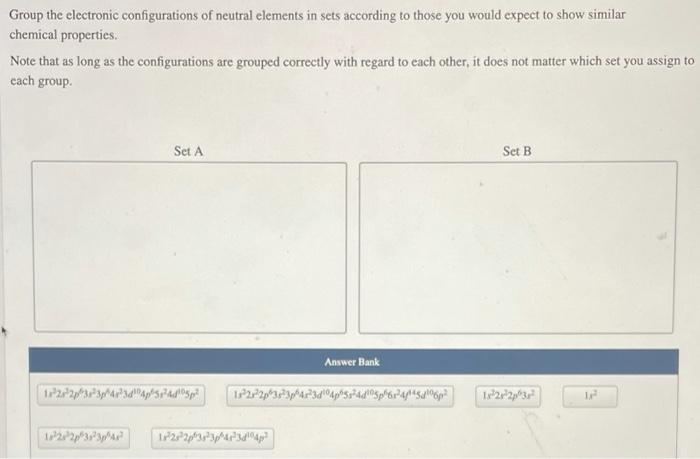
Groupings of Elements, Group the electronic configurations of neutral elements in sets
Based on their electronic configurations, elements can be grouped into various sets. One common grouping is the noble gases, which have a full valence shell and are highly unreactive. Another grouping is the alkali metals, which have a single valence electron and are highly reactive.
Halogens, with one valence electron less than a full valence shell, are also grouped together due to their high reactivity.
Noble Gases
The noble gases, including helium, neon, argon, krypton, xenon, and radon, have a unique electronic configuration with a full valence shell. This configuration makes them highly stable and unreactive, as they have no tendency to gain or lose electrons.
Alkali Metals
The alkali metals, including lithium, sodium, potassium, rubidium, cesium, and francium, have a single valence electron in their outermost energy level. This configuration makes them highly reactive, as they readily lose their valence electron to achieve a stable octet configuration.
Halogens
The halogens, including fluorine, chlorine, bromine, iodine, and astatine, have seven valence electrons. This configuration makes them highly reactive, as they tend to gain one electron to achieve a stable octet configuration.
Transition Metals
Transition metals, which include elements in Groups 3-12, have partially filled d orbitals in their electronic configurations. These d electrons are responsible for the unique properties of transition metals, such as their variable oxidation states and ability to form complex ions.
Questions and Answers
What are the benefits of grouping electronic configurations?
Grouping electronic configurations allows for the identification of periodic trends, prediction of chemical properties, understanding of reactivity patterns, and the development of models for atomic and molecular behavior.
How do you determine the electronic configuration of an element?
The electronic configuration of an element can be determined using the periodic table, which provides the atomic number and electron configuration for each element.
What is the significance of noble gases in the context of electronic configurations?
Noble gases have stable electronic configurations with a full outermost shell, making them unreactive and chemically inert.