Embark on a journey into the realm of chemistry with our Simple Binary Ionic Compounds Worksheet, meticulously crafted to provide a comprehensive understanding of these fundamental building blocks of matter. This worksheet delves into the formation, properties, and applications of ionic compounds, equipping you with a solid foundation in this essential topic.
As we delve deeper into the intricacies of ionic compounds, we will explore their unique characteristics, including their electrostatic nature, high melting and boiling points, and solubility in water. We will also investigate the fascinating world of simple binary ionic compounds, learning how to name and identify these compounds based on their elemental components.
Ionic Compounds: Simple Binary Ionic Compounds Worksheet

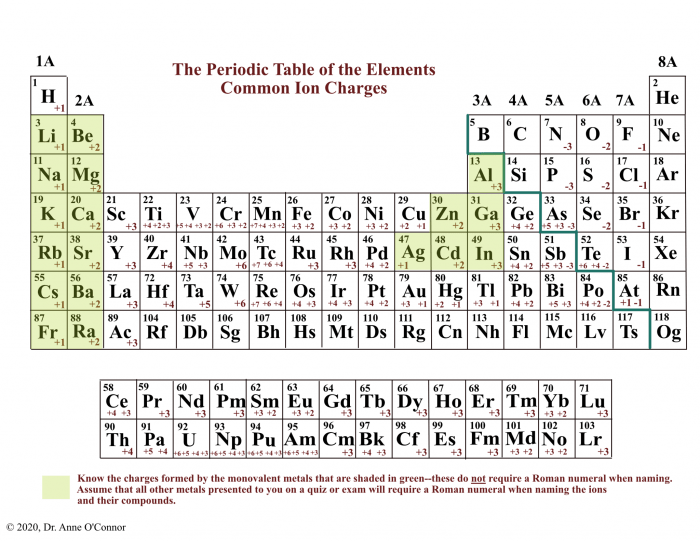
Ionic compounds are formed when a metal loses one or more electrons to a nonmetal. The metal becomes a positively charged ion, called a cation, and the nonmetal becomes a negatively charged ion, called an anion. The oppositely charged ions are attracted to each other by electrostatic forces, forming an ionic bond.Ionic
compounds typically have high melting and boiling points, are hard and brittle, and are good conductors of electricity when dissolved in water. They are also soluble in water, forming ions that can conduct electricity. Examples of ionic compounds include sodium chloride (NaCl), potassium iodide (KI), and calcium oxide (CaO).
Simple Binary Ionic Compounds
Simple binary ionic compounds are ionic compounds that contain only two elements, a metal and a nonmetal. The metal is always written first in the chemical formula, followed by the nonmetal. The charges of the ions are indicated by Roman numerals.
For example, the chemical formula for sodium chloride is NaCl, indicating that the sodium ion has a charge of +1 and the chloride ion has a charge of
1.
Simple binary ionic compounds are named using the following rules:
- The name of the metal is written first, followed by the name of the nonmetal.
- The name of the nonmetal ends in
ide.
- The charge of the metal ion is indicated by a Roman numeral in parentheses after the metal’s name.
For example, the chemical formula for iron(III) oxide is Fe2O3, indicating that the iron ion has a charge of +3 and the oxide ion has a charge of
2.
Worksheet, Simple binary ionic compounds worksheet
*Practice Problems
1. Write the chemical formula for the following ionic compounds
Sodium chloride
Potassium iodide
Calcium oxide
Iron(III) oxide
2. Name the following ionic compounds
NaCl
KI
CaO
Fe2O3
Answer Key
- 1.
- NaCl
- KI
- CaO
- Fe2O3
- 2.
- Sodium chloride
- Potassium iodide
- Calcium oxide
- Iron(III) oxide
Applications
Simple binary ionic compounds have a wide variety of applications in everyday life. For example, sodium chloride is used as a food additive, potassium iodide is used to prevent iodine deficiency, and calcium oxide is used in the production of cement.
Safety
Simple binary ionic compounds can be harmful if ingested or inhaled. It is important to wear gloves and a mask when working with these compounds. They should also be disposed of properly according to local regulations.
Key Questions Answered
What are ionic compounds?
Ionic compounds are chemical compounds formed by the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions).
How are simple binary ionic compounds formed?
Simple binary ionic compounds are formed when a metal loses one or more electrons to a nonmetal, resulting in the formation of positively charged metal ions and negatively charged nonmetal ions.
What are the properties of ionic compounds?
Ionic compounds typically have high melting and boiling points, are soluble in water, and conduct electricity when dissolved or molten.
What are some applications of simple binary ionic compounds?
Simple binary ionic compounds are used in a wide variety of applications, including as table salt (NaCl), baking soda (NaHCO3), and plaster of Paris (CaSO4).