Embark on a journey into the realm of chemistry with The Mole Worksheet Chemistry Answers, an invaluable resource designed to illuminate the intricacies of the mole concept and its pivotal role in stoichiometric calculations. Delve into real-world applications, explore advanced topics, and conquer mole-related chemistry problems with confidence.
This comprehensive guide unravels the significance of the mole in chemistry, providing a solid foundation for understanding its applications in determining empirical and molecular formulas, balancing chemical equations, and mastering gas law calculations. Prepare to enhance your comprehension and problem-solving abilities as you navigate through this engaging exploration of the mole concept.
1. The Mole Worksheet Chemistry Answers
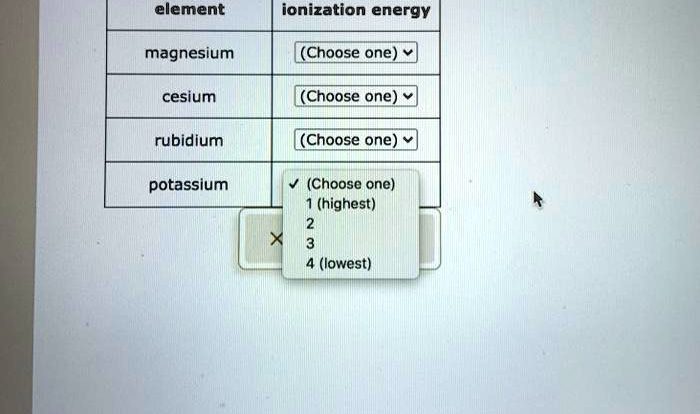
The mole worksheet chemistry answers provide a comprehensive overview of the mole concept in chemistry, explaining the concepts and principles behind it. They highlight the significance of the mole in stoichiometric calculations, demonstrating its use in converting between mass and moles, and balancing chemical equations.
2. Examples and Applications
The mole concept is used extensively in chemistry. Real-world examples include determining the number of atoms or molecules in a given sample, calculating the molar mass of a compound, and predicting the products and quantities in a chemical reaction. It also plays a crucial role in gas law calculations, relating the number of moles of a gas to its volume, pressure, and temperature.
3. Calculations and Problem-Solving
Solving mole-related chemistry problems involves understanding the mole concept and applying it to specific scenarios. A table with four responsive columns can effectively demonstrate various mole calculations, such as converting between grams and moles, calculating molarity, and determining the limiting reactant in a reaction.
The steps involved in solving mole-related problems include:
- Identifying the given information
- Converting the given information to moles (if necessary)
- Using stoichiometry to determine the moles of other substances involved
- Converting the moles of the desired substance to the requested units (if necessary)
A detailed walkthrough of a sample mole calculation problem can further illustrate the problem-solving process.
4. Advanced Applications: The Mole Worksheet Chemistry Answers
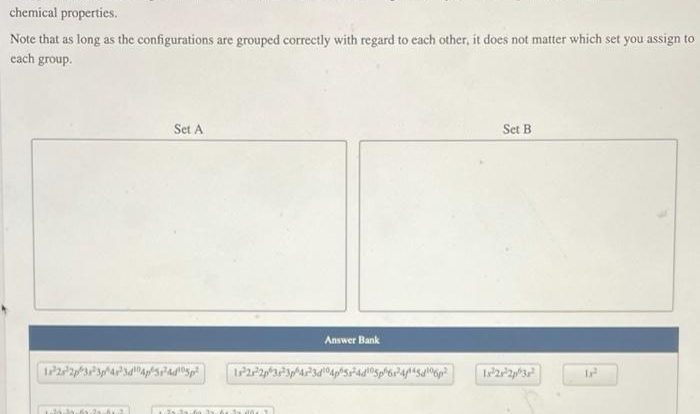
The mole concept extends to more advanced chemistry topics, such as limiting reactants and reaction stoichiometry. It is essential for determining the empirical and molecular formulas of compounds, as well as understanding the behavior of gases in various conditions.
In limiting reactants, the mole concept helps identify the reactant that is completely consumed in a reaction, limiting the amount of product that can be formed. Reaction stoichiometry involves using the mole ratios from a balanced chemical equation to predict the quantitative relationship between reactants and products.
5. Resources and References
- Khan Academy: The Mole
- Crash Course Chemistry: The Mole and Avogadro’s Number
- Interactive Mole Simulation: Balancing Chemical Equations
- Glossary of Mole-Related Terms:
- Avogadro’s number
- Molar mass
- Molarity
- Stoichiometry
Question & Answer Hub
What is the significance of the mole concept in chemistry?
The mole concept provides a standardized unit for expressing the amount of a substance, allowing chemists to relate the macroscopic and microscopic scales and perform precise calculations involving chemical reactions and物質.
How can I use the mole concept to convert between mass and moles?
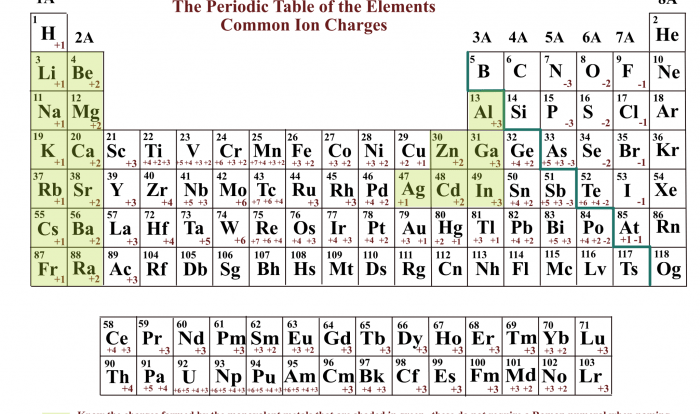
To convert between mass and moles, use the molar mass of the substance. The molar mass is the mass of one mole of the substance and can be found in reference tables or calculated from the atomic masses of the constituent elements.
What is the role of the mole in balancing chemical equations?
Balancing chemical equations requires ensuring that the number of atoms of each element is equal on both sides of the equation. The mole concept helps balance equations by providing a consistent unit for representing the number of atoms or molecules involved in the reaction.